BrightPoint: A Closer Look
Lumoptik, Inc is a medical device company that has developed the BrightPoint™ needle guidance system to assist physicians with needle placement during epidural procedures for childbirth, spine/back pain and surgical procedures such as knee and hip replacements. Over 11 million epidural procedures are performed in the US each year. The large majority of these procedures are performed free hand by physicians with no mechanical or electronic needle guidance. Complications are common.
Below is a quote from the New England Journal of Medicine in July 2014:
“Epidural injections are common. An estimated 10 million to 11 million injections (2.2 million in the Medicare population) are administered annually in the United States. Although many injections are used for indications other than spinal stenosis, epidural injections have become almost an expected part of a comprehensive non-operative treatment protocol in patients with this condition.”
Epidural procedures are one of the fastest growing procedures in the US and worldwide with an estimated 30 million worldwide procedures. Over $5 billion is spent on epidural injections in the US alone.
Below is a quote from the Journal of Anesthesiology and Clinical Pharmacology in March 2017:
“Although epidural analgesia is widely used for pain relief, it is associated with a significant failure rate. Loss of resistance technique, tactile feedback from the needle, and surface landmarks are traditionally used to guide the epidural needle tip into the epidural space (EDS).”
The current epidural procedure using the loss of resistance technique has remained largely unchanged since it was introduced in the 1920’s. The procedure has a long learning curve for new practitioners. It is generally recognized that it takes 50 to 100 patient procedures before a new practitioner is reasonably skilled to perform the procedure without the guidance of a senior physician. The BrightPoint™ Needle Guidance System is designed to be an easy to use, low cost, effective tool for training and reducing complications from incorrect needle placement during epidurals. Lumoptik plans to introduce BrightPoint™ through hospital teaching programs. There are approximately 1,170 teaching hospitals in the US. This represents about 21% of the total hospitals in the US.
Lumoptik believes that there is a large opportunity for needle guidance products in this large, rapidly expanding market. The company has moved past the prototype stage and is now beginning to manufacture devices for FDA submission testing, conducted multiple animal studies proving the device works, filed a patent to protect the intellectual property (more are planned) and hired an experienced medical device CEO to guide the company. The company is currently contacting the business development teams at the major needle manufacturers to begin developing distribution relationships.
A brief overview of the market opportunity:
Large Market
There are over 11 million epidural procedures performed in the US each year. It is one of the fastest growing of all medical procedures. This is not limited to the US. Epidural steroid injections are the most commonly performed procedure for pain relief in the world. Western Europe is estimated to have a similar number of procedures to the US. Worldwide Epidural procedures are estimated over 30 million per year.
Market Need
The standard of care is for physicians perform the procedure free hand, without guidance using the Loss of Resistance (LOR) technique. Incorrect needle placement, side effects and complications are common. It is especially difficult to train new physicians (residents and nurse anesthetists) how to perform epidurals because the procedure is 100% done by hand and the trainer cannot place their hands on the needle during the training procedures.
Market Need Not Being Met
Cost is an issue, as the number of procedures has exploded, insurance reimbursement is under increased scrutiny. Ultrasound and fluoroscopic guidance involve expensive capital equipment purchases, are not well reimbursed and used in less than half of the procedures. There are currently no other low cost, easy to use alternative real-time needle guidance methods available to physicians at this time.
Lumoptik Solution
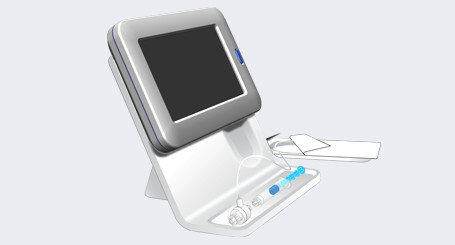
The BrightPoint™ Needle Guidance System is designed to be a low cost, easy to use device composed of a reusable handheld controller plus a single use, disposable fiber optic light channel designed to fit in the lumen of existing epidural needles. This is a razor (reusable handheld controller) and razor blade (disposable fiber optic) commercial design.
- BrightPoint™ operates based on the changes in light reflection from different tissue types at the tip of the needle. The color of the tissue at the tip of the needle is shown on the controller screen along with a scrolling line graph of the relative reflectance of the tissue at the tip of the needle—the line moves to the bottom of the chart for darker tissue and top of the chart for lighter tissue.
- As the tip of the needle penetrates in to different tissue (skin, fat, muscle, connective, ligamentum flavum and epidural space) the reflection changes providing the physician with real-time information on the location of the tip of the needle. Upon penetrating the ligamentum flavum and entering in to the epidural space, the reflection makes a distinct change from the whitish ligament color to the blackish reflection of the epidural space. On the controller screen, the tissue color will show the change from white ligament to the dark epidural space and the scrolling line chart will show a significant drop from the high reflectance of the white ligament to the low reflectance of the dark epidural space.
- The lumoptik device is an addition to, not a replacement for, the manual (unguided) Loss of Resistance (LOR) method currently used by anesthesiologists. It does not require any changes to the LOR method and works with the current needles and syringes.
- The BrightPoint™ Epidural system is designed to provide real-time visual guidance for training procedures with the aim to reduce the number of training procedures, reduce complications and increase successful procedures.
Platform Technology
The company believes that the Lumoptik technology has multiple high-value medical uses beyond epidural procedures. These uses include:
- Urology catheter placement
- Orthopedic access for pedicle screws and other procedures
- Vascular access
- Cardiac catheter introduction
- Ophthalmic treatments
- Veterinary use
Management plans to develop the next generation product after launching the epidural needle guidance product. A pipeline of products is intended to increase the value of the company and help attract large acquirers. The epidural needle guidance product was chosen as the company’s first due to the market size, need, access and regulatory timing.